About The Study
Before emerging therapies can be approved for widespread public use, they must first be tested in controlled clinical trials like this one. By joining the EVO756 study, participants are playing a vital role in helping doctors and scientists make meaningful progress in the field of chronic spontaneous urticaria (CSU) research, and helping to better understand conditions tied to inflammatory itch.
What Are The Benefits?
Study participants will have access to a team of physicians and research scientists who understand their unique situation and can help answer questions and address concerns. Throughout the trial, patients will be monitored in a professional clinical environment and participate in scientific innovation.
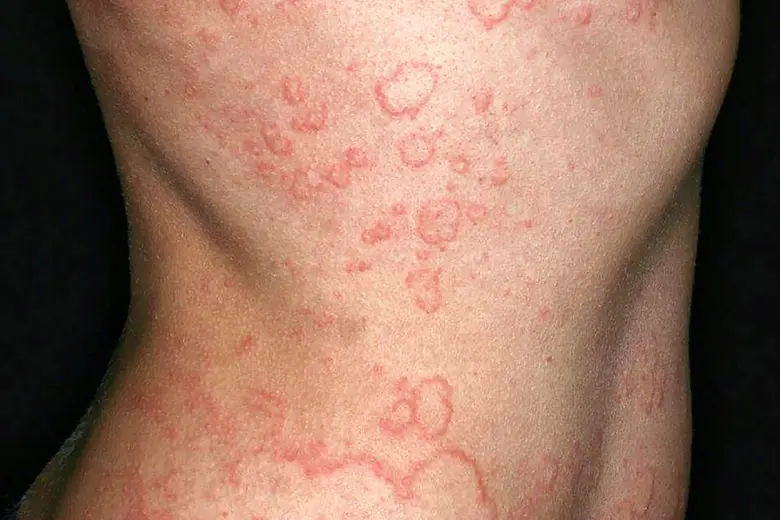

What Is Being Tested?
The main purpose of this study is to evaluate the efficacy of an experimental drug called EVO756 in adults with moderate to severe CSU, and how safely it interacts with the body. During the clinical trial, the research team will be studying whether blocking a receptor (called MRGPRX2) on immune cells (specifically mast cells), can help with chronic hives by preventing those immune cells from undergoing a process in which the body releases histamine and other inflammatory mediators. In CSU patients, that histamine-releasing process, called degranulation, is what is tied to itching and discomfort. Read our FAQs to learn more about this study, and what to expect as a participant.
What Patients Are Saying
Clinical Trials Make A Difference
When you participate in a clinical study, you provide valuable information that could eventually lead to a better treatment and—with the right breakthrough—to better outcomes. Take our brief survey to see if you are a good fit. There is no obligation.
Participate In Innovation
Shape The Future
Help Others
Still have questions?
Contact us anytime at help@clinicalenrollment.com.






