How It Works
Starting the process only takes a few minutes
Complete The Survey
Answer some brief, easy questions and we’ll let you know whether you may be eligible to participate in the study.
Schedule An Appointment
If you qualify, the next step is speaking with a team member. Just find 10 – 15 free minutes in your day and we’ll get you connected.
Speak To A Team Member
Our dedicated specialists understand your situation and will listen, answer any questions, and address your concerns. We’re here to help.
Who Can Participate?
Here are the qualifications participants need to meet in order to join the chronic spontaneous urticaria (CSU) study.
- Are at least 18 years old
- Have had a confirmed diagnosis of CSU for three months or more
- Do not have a history of diseases with hives or swelling symptoms, such as urticarial vasculitis, erythema multiforme, cutaneous mastocytosis (urticaria pigmentosa), hereditary or acquired angioedema, or any other skin disease that may impede the study team’s ability to assess
- If taking antihistamines, participant must be on a stable regimen for at least four weeks prior to screening commencing, and throughout the study
Learn more about our participation criteria to see if you may be a good fit for this trial.
Understanding urticaria
What exactly is chronic spontaneous urticaria (CSU)?
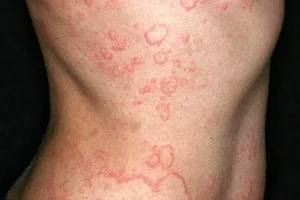
There are multiple forms of urticaria, but chronic spontaneous urticaria (CSU) is defined as hives that come and go unexpectedly, with their root cause unknown. These hives—or raised, red, itchy bumps or welts on the skin—cause discomfort in sufferers, and occur frequently (almost daily) for a duration of six weeks or more. They typically last between 30 minutes and 24 hours. Additionally, with CSU, hives may be big or small, occur anywhere on your body, the affected area may be warm to the touch, and in more severe cases, may cause swelling, headache, fatigue, joint pain, wheezing, diarrhea and/or rapid heartbeat.
How is it treated?
What We Hope To Do
More than 500,000 Americans are estimated to suffer from chronic urticaria. The spontaneous form is particularly frustrating, as what causes patients’ to break out in hives is unknown.2
Frequently Asked Questions
What are the symptoms of chronic spontaneous urticaria?
Signs may include:3
- Raised, red bumps or welts on the skin
- Hives that last between 30 minutes and 24 hours, but occur most days of the week and persist for a period of 6 weeks or more
- Itchiness (pruritus) that can be intense
- Swelling of the affected skin area
- Welts that vary in size, change shape, and appear and fade repeatedly
- Headache
- Joint Pain
- Wheezing
- Rapid Heartbeat
3. Source: https://www.mayoclinic.org/diseases-conditions/chronic-hives/symptoms-causes/syc-20352719
Who may be a good candidate to apply for this study?
- Are at least 18 years old
- Have had a confirmed diagnosis of CSU for at least three months
- Do not have a history of diseases with hives or swelling symptoms, such as urticarial vasculitis, erythema multiforme, cutaneous mastocytosis (urticaria pigmentosa), hereditary or acquired angioedema, or any other skin disease that may impede the study team’s ability to assess
- If taking antihistamines, participant must be on a stable regimen for at least four weeks prior to screening commencing (and throughout the study)
What will happen during the study?
This study is randomized and double-blind, which means neither participants nor study staff will know whether participants receive the investigational treatment, EVO756, in varying dose levels, or a placebo, which contains no active medication. Participants will receive either the study drug or placebo in tablet form to take for 12 weeks and be evaluated on an out-patient basis. Total duration of participation for each subject is expected to be approximately 18 weeks, including up to 30 days for screening, the 12-week treatment window, and two subsequent weeks of follow-up.
Are there any costs to participate in this study?
Still have questions?
Contact us anytime at help@clinicalenrollment.com.
